Brief introduction
Structure and function of the heart
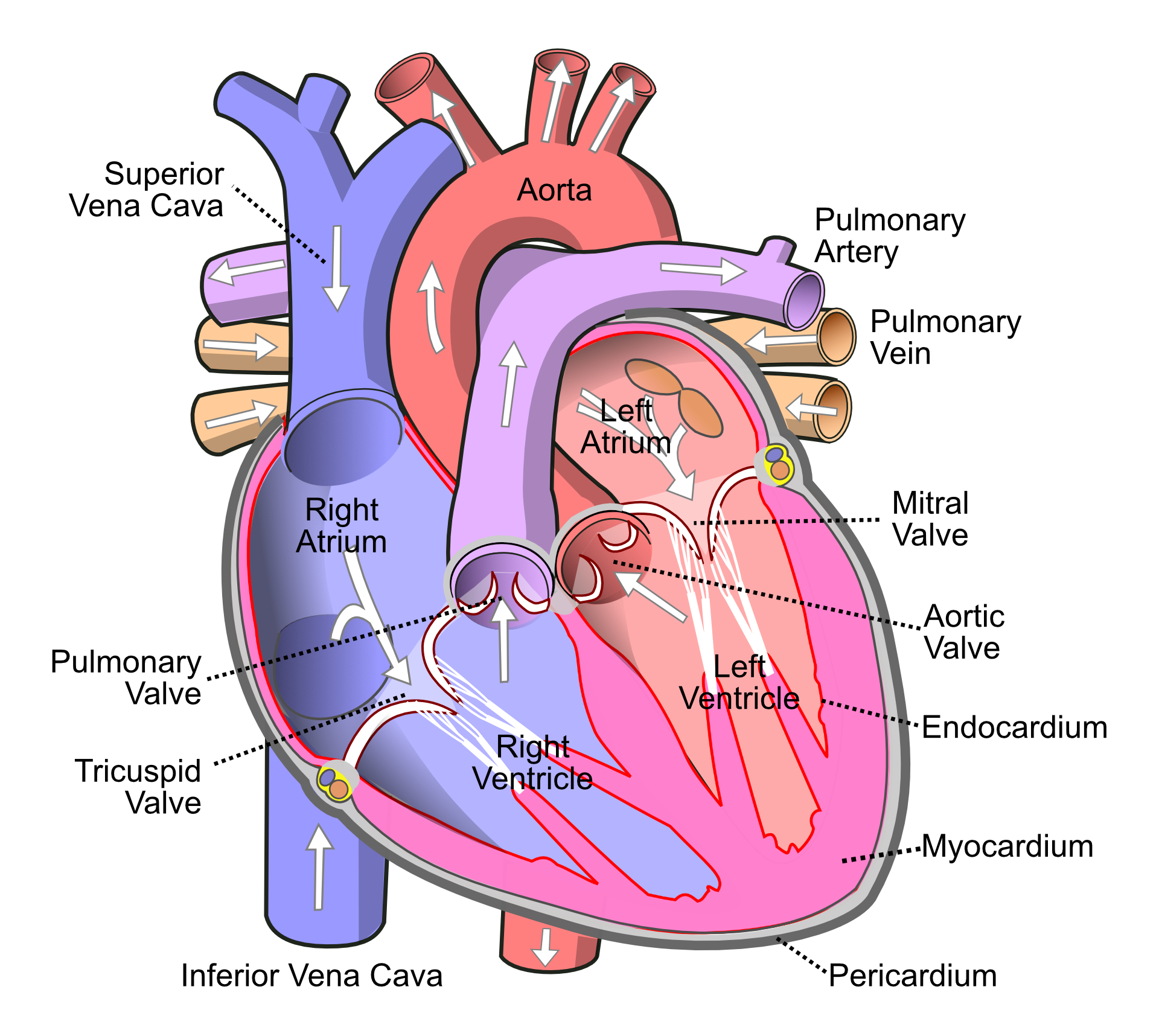
The heart has 4 hollow chambers - 2 atria and 2 ventricles. The atria are smaller with thinner walls and hold blood transiently while the ventricles would be in contracted state unable to accept more blood; this contracted state of the ventricles is called 'systole'. The right side of heart (right atrium and right ventricle) receives deoxygenated blood from rest of the body. The right ventricle sends blood to the lungs, which remove carbon dioxide from the blood and replenish the spent oxygen. From lungs, the blood sequentially reaches the left atrium and left ventricle. Left ventricle (LV) is the largest chamber of the heart with strongest ability to contract, which in turn pumps blood into the aorta (the largest artery). Most of the present writeup will focus on the LV.
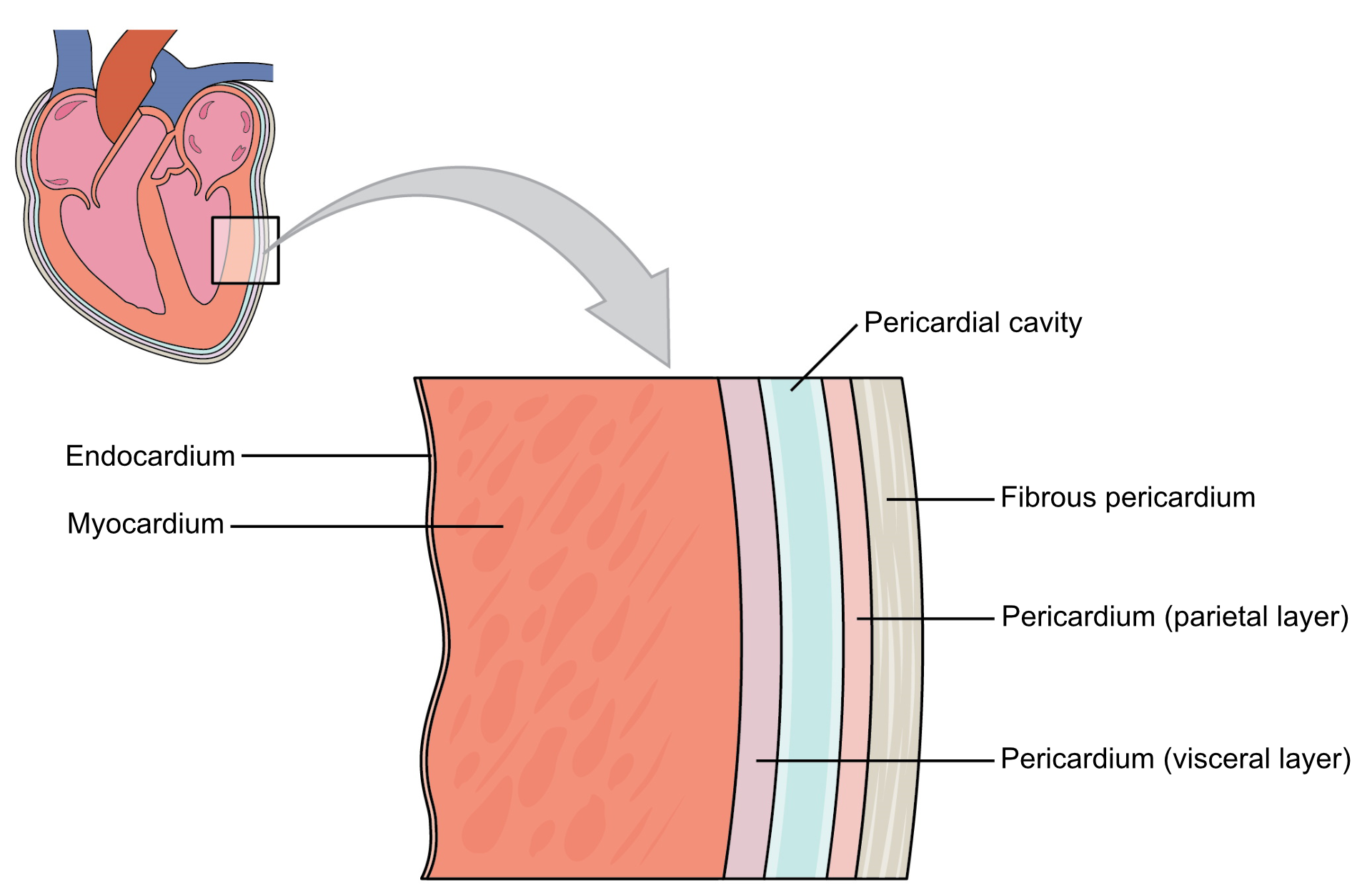
Layers of the heart wall
The heart wall has 3 major layers. From outside to inside, they are: pericardium, myocardium and endocardium. Myocardium is the thickest layer and consists almost entirely of the muscles that are responsible for its contraction. The individual cells of the myocardium are called cardiomyocytes.
Most of the discussions about function and disorders of the heart are focused on left ventricular myocardium.
Energy requirement of the heart
Heart muscles keep on contracting throughout the day even while we would be resting. Hence, myocardium has high oxygen requirement (to produce energy needed for muscle contraction). Cardiomyocytes can utilize both glucose as well as fatty acids for energy production. Utilization of fatty acids yields more energy but also consumes more oxygen. Glycolysis (one of the earlier steps in glucose break down) yields less energy with lesser consumption of oxygen, but is much quicker. Hence, cardiomyocytes tend to preferentially use glucose in cases of sudden increase in workload and/or relative deficiency of oxygen.
Blood supply of the heart (myocardium)
The blood vessels carrying oxygenated blood to the myocardium are called 'coronary arteries'. There are 3 main coronary arteries: left anterior descending (LAD), left circumflex (LCx) and right coronary (RCA). Of these the first 2 branch out from a very short artery (< 1 cm long) called 'left main coronary', which in turn arises from the lower most part of aorta ('root of aorta') as it emerges from the LV. RCA arises directly from the aorta at the same level.
As the names suggest LAD and LCx predominantly supply the left side of the heart (including the LV), and hence, their abnormalities are more likely to be symptomatic, and warrant greater attention than that of the RCA.
Branches of coronary arteries and their properties
The 3 major coronary arteries as well as their branches (before they pierce the myocardium) are called 'epicardial arteries'. Under normal circumstances, they permit high flow of blood without offering much resistance. So, the rate of blood flow through them is largely determined by their diameter (somewhat constant), force with which the LV pumps blood (variable), and frequency of these contractions or 'heart rate' (variable).
After piercing into the myocardium, the branches of epicardial arteries are respectively called 'subepicardial', 'intramural' and 'subendocardial' arteries. Of these, the intramural arteries are capable of offering a very wide range of mechanical resistance to blood flow by changing their diameter (pressure of flowing blood drops by 16 times when diameter of a blood vessel halves). 'Autoregulation' refers to this ability to dynamically alter the flow of blood. The subendocardial arteries offer relatively high and constant resistance over time. Blood flowing into the subepicardial, intramural and subendocardial arteries enters into much smaller blood vessels called 'arterioles' and further into 'capillaries'. Capillaries have semi-porous walls through with exchange of solutes (like glucose and salts) and gases (like oxygen and carbon dioxide) occurs with the extracellular fluid (ECF). The ECF in turn exchanges these with the individual cardiomyocytes in a process simplistically called as 'perfusion'.
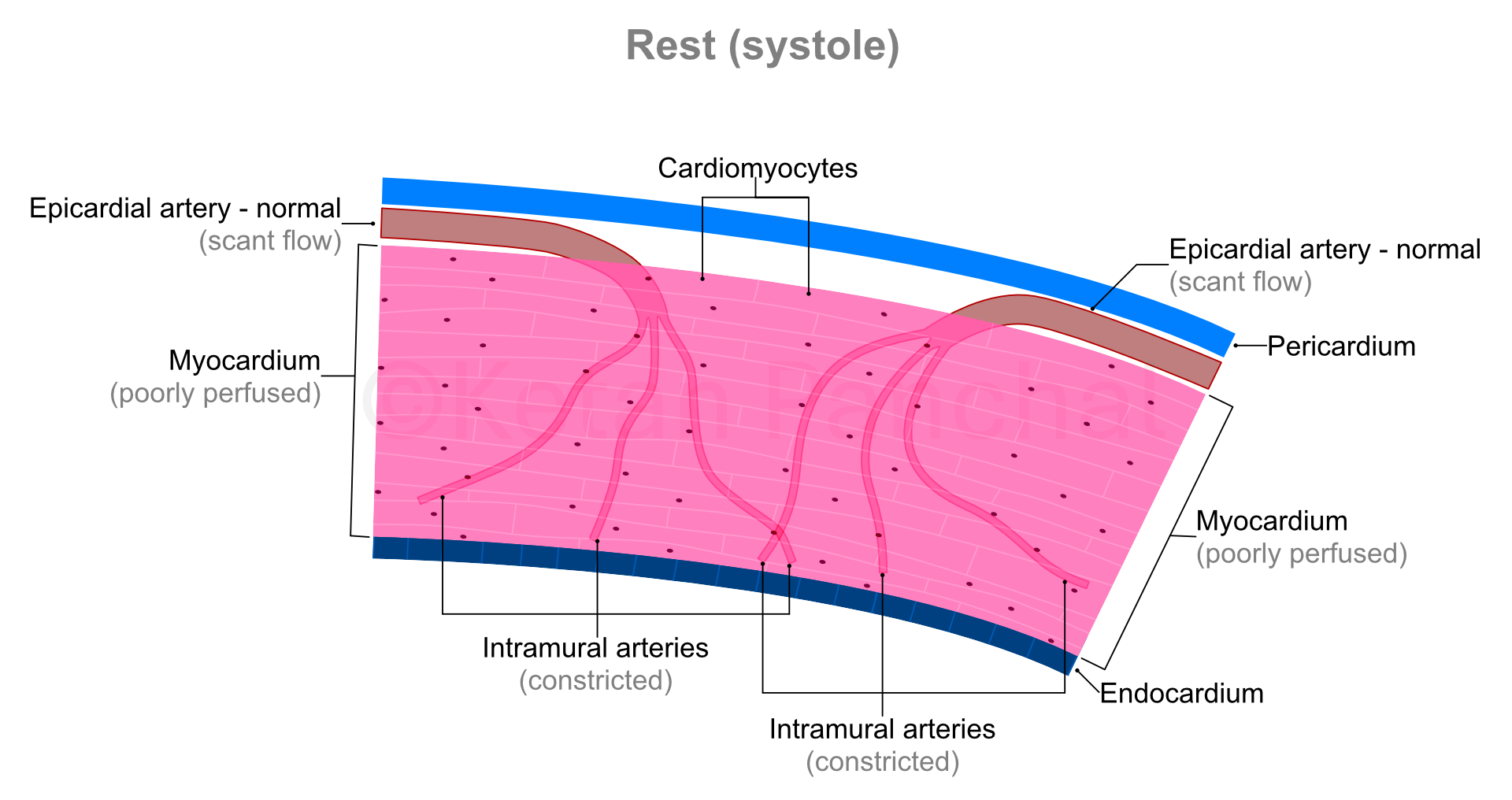
In a person with normal heart function (normal strength of LV contraction) and without narrowing of epicardial arteries, flow of blood across them is much more than needed by the cardiomyocytes. So, the intramural arteries remain suitably narrowed (constricted) to limit blood flow.
Cardiac cycle and impact on blood supply
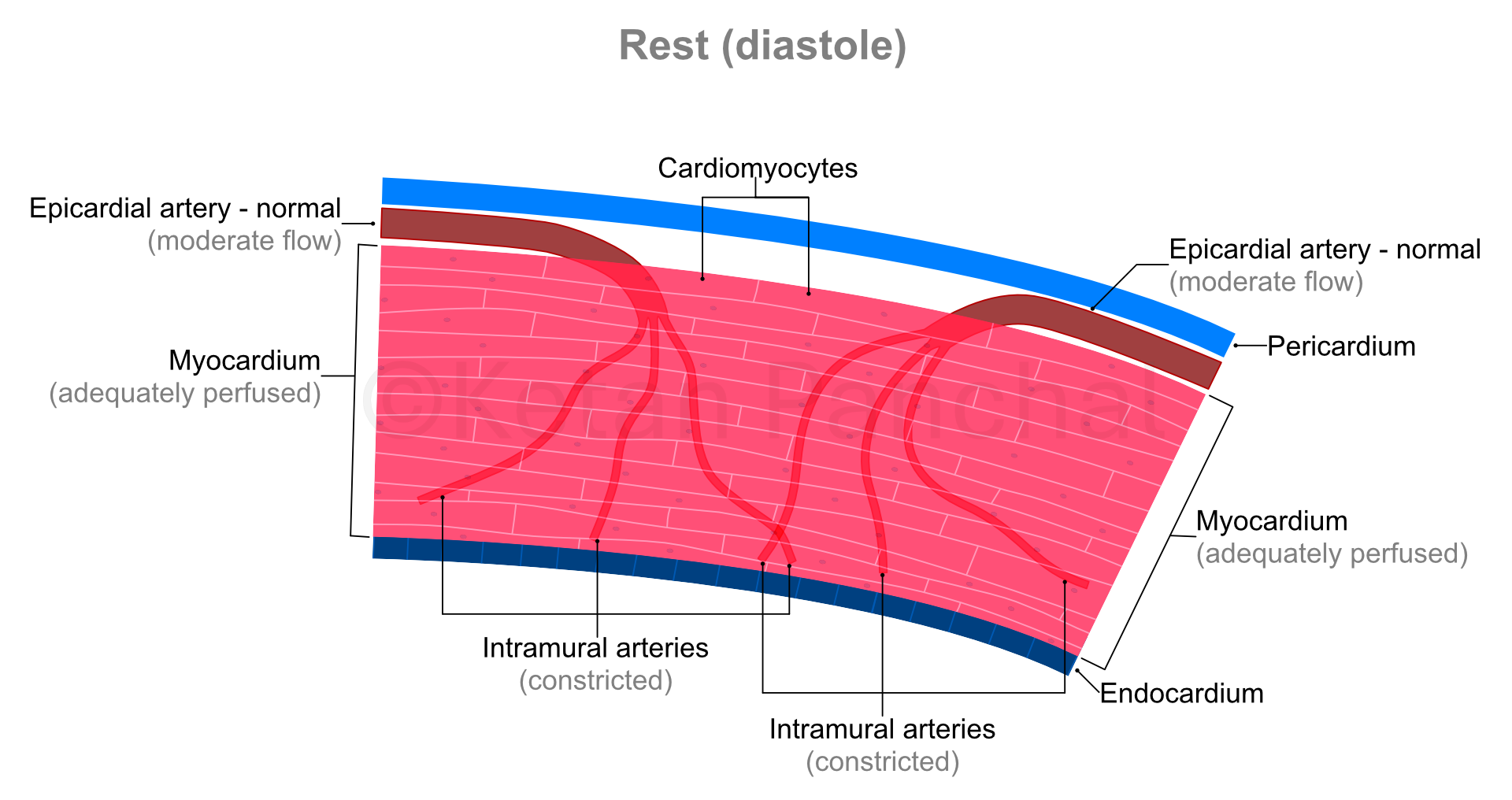
Cardiac cycle is a set of motions of heart involving one contraction and one relaxation, wherein the heart returns to its initial state at the end of the cycle. In simple terms, one cardiac cycle is one 'heartbeat'. The process of contraction is called 'systole', during which blood is pushed out of the LV into the aorta. 'Diastole' is the process of relaxation when the LV gets filled by the blood released from the left atrium. As the heart rate increases, the cardiac cycle shortens in time. E.g., with a heart rate of 75 beats per minute, each cardiac cycle lasts 0.8 second.
Even when the heart rate changes, the absolute duration of systole tends to remain constant (at ~0.3 second). So, at low heart rates, diastole tends to be longer than systole.
During systole, the intramural arteries are squeezed between the heart muscles, and blood flow across them gets restricted. Most of the blood flow into the myocardium happens during diastole.
Impact of rest and stress on myocardial blood supply
'Stress' in present context refers to physical stress, wherein because of vigorous use of large muscles of the body (e.g., that of lower limbs), oxygen and glucose requirement of the body increase, and carbon dioxide needs to be eliminated at a faster rate. Heart responds to these requirements by contracting more forcefully (increasing the amount of blood ejected into the aorta during each systole) and contracting more frequently (thus increasing the heart rate). Consequently, the heart muscles also require more oxygen and glucose, and by extension greater flow of blood.
As the cardiac cycle shortens so does the duration of diastole (which is when the blood actually reaches into the heart muscles - as explained above). So, in background of increased need for blood, the heart muscles paradoxically experience shorter durations of blood supply! This problem is overcome by dilatation (widening) of the intramural arteries. Even minor widening of these arteries greatly reduces the offered resistance with resultant marked increase in blood reaching the heart muscles. The ability of the arterial system of the myocardium to increase blood supply in times of increased demand by dilating is known as 'coronary flow reserve' (CFR).
| Systole | Diastole | |
|---|---|---|
| Rest |
|
|
| Stress |
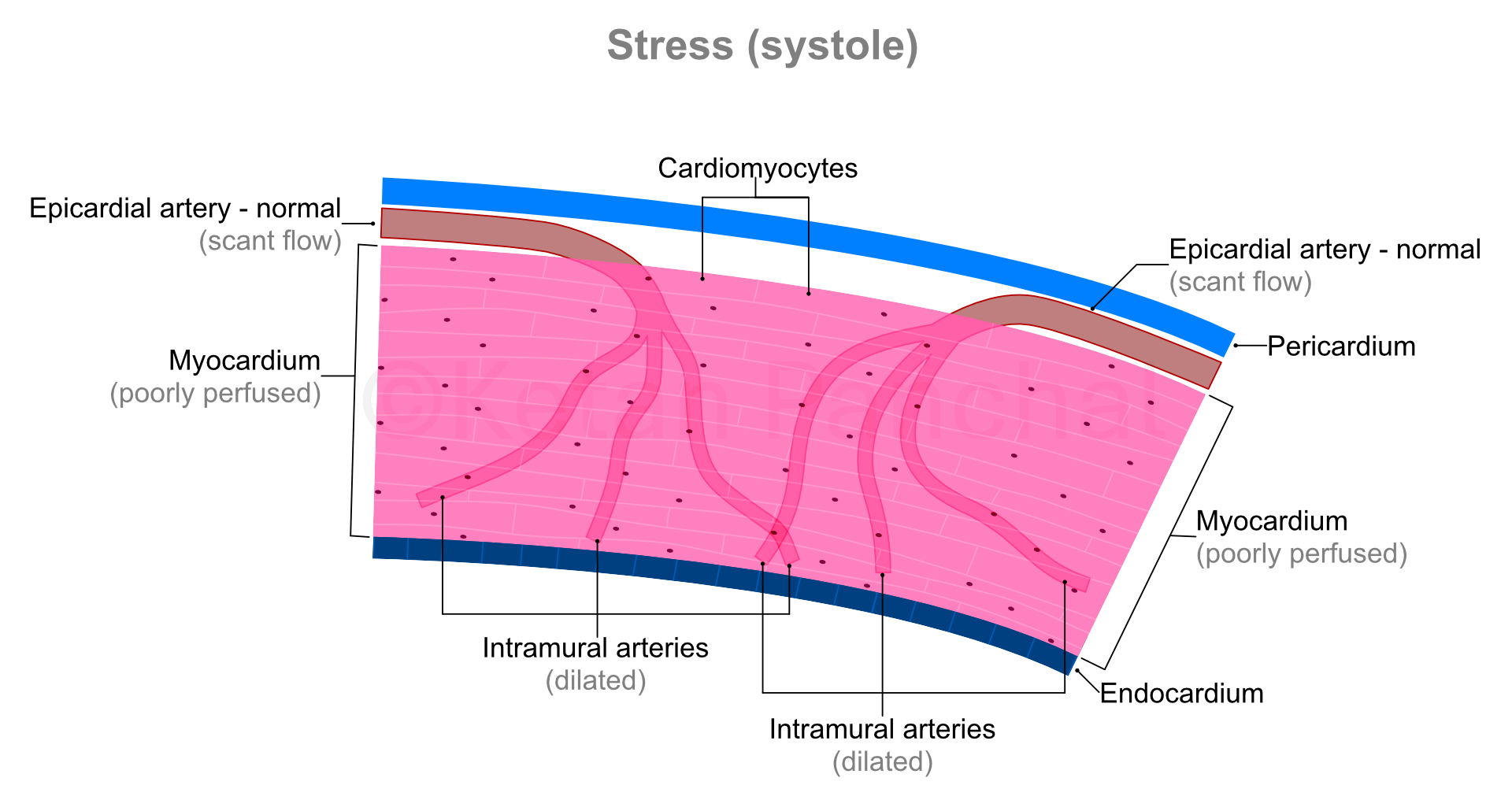
|
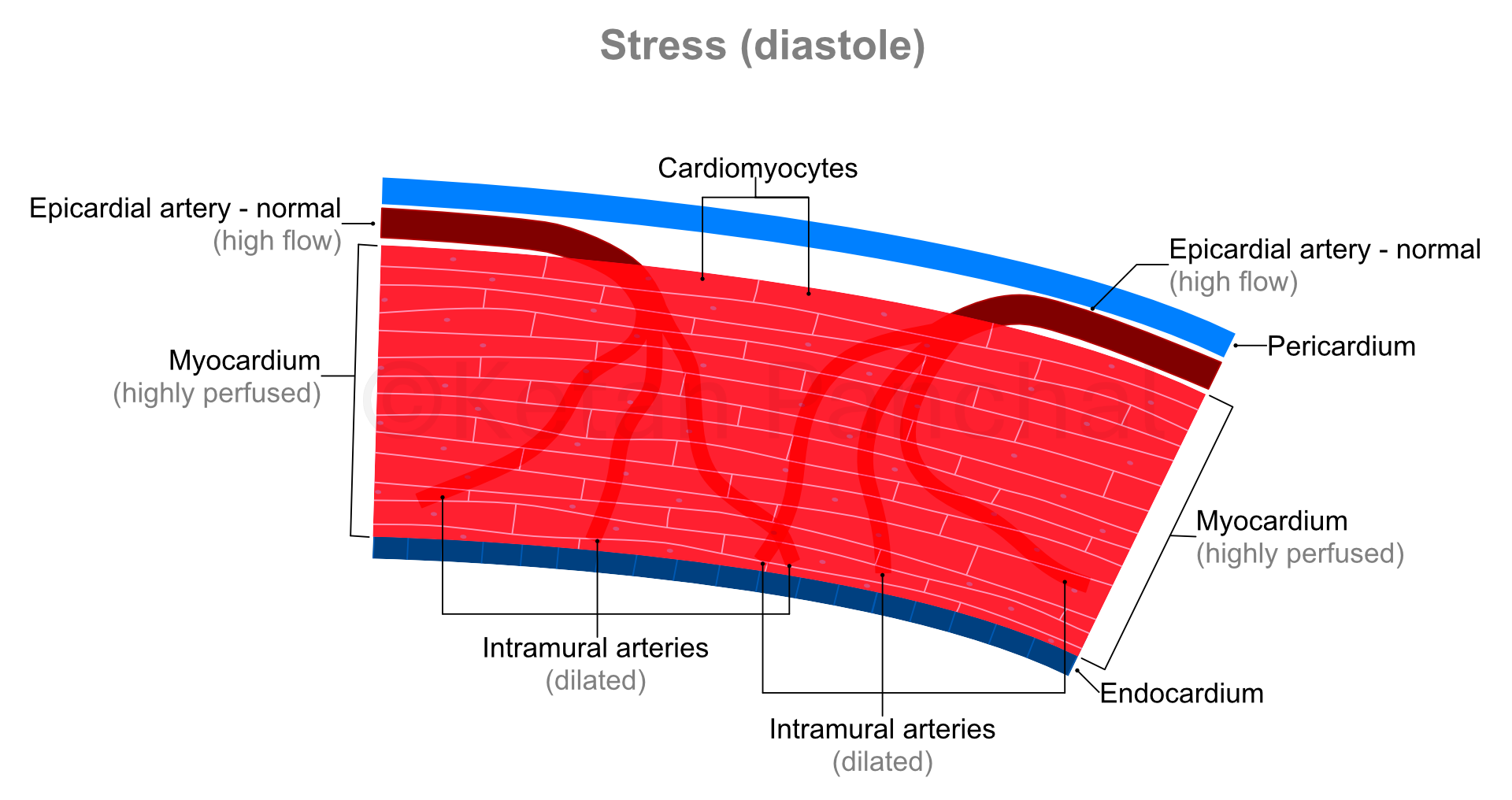
|
Coronary artery disease
'Coronary artery disease' (CAD) refers to the narrowing of lumen (the hollow internal part) of epicardial coronary arteries. These narrowings are also known as 'stenosis' or 'occlusion'. The most common cause of CAD is atherosclerosis, which in turn is a process characterized by build up of abnormal material (usually fats) and abnormal proliferation of cells within the walls of an artery. The abnormal material and cells together are called 'atheroma' or 'atheromatous plaque'. When these plaques become large enough, they start indenting through the inner most layer of cells ('endothelial cells') into the lumen of the artery reducing its effective diameter and cross-sectional area.
Inadequate blood reaching myocardium (for a given workload) is known as 'ischaemia', and similarly demand-supply mismatch of oxygen is known as 'hypoxia' (which for our discussion go hand-in-hand).
A reduction in diameter of coronary arteries of up to 50%, or of cross-sectional area of 70% is usually needed for there to be detectable reduction in blood supply through the epicardial arteries. From 70% to 90% narrowing, autoregulation (in form of dilatation of intramural arteries) ensures adequate perfusion at rest especially because of low myocardial oxygen demand. However, during stress (raised oxygen demand), even maximal dilatation of intramural arteries proves insufficient. Beyond 90% narrowing, autoregulation proves insufficient even at rest with ensuing permanent ischaemia.
Harms caused by CAD
Harms caused by CAD can be roughly categorized into 2 categories: (a) Gradual response of the myocardium to low-level ischaemia. (b) Rapid-onset events that cause severe morbidity (e.g., myocardial infarction [MI] or sudden death).
Gradual-onset harms of CAD
Gradually developing myocardial responses to CAD are termed 'ischaemic heart disease' (IHD). They develop over months to years, and often remain undetected because of lack of obvious symptoms. In simple words, CAD is the narrowing of blood vessels, and IHD is the resultant reduction in blood supply to the myocardium and accompanying (undesirable) consequences.
When ischaemic episodes are mild and short-lived, the responses in myocardium are completely reversible, and in fact protective. These changes (stunning and/or short-term hibernation) involve reduced contractility of the affected part of myocardium in order to reduce the oxygen demand. These changes can be completely reversed if blood supply is normalized.
More severe and/or prolonged ischaemia elicits harmful responses in the myocardium (chronic stunning or hibernation). These changes can only be partially reversed even with restoration of blood supply or could be entirely irreversible. Reduced or lost ability to contract in one part of the myocardium results in increased stress (and oxygen demand) in other parts of better or normally perfused myocardium. The myocardium facing chronic ischaemia stiffens (remodelling), which results in further reduced blood supply in the vicinity. The 'junctional tissue' transmitting electrical impulses that initiate contraction of heart muscles also malfunctions in face of ischaemia; this results in asynchronous and overall inefficient contraction of heart muscles ('arrhythmia') reducing blood supply to all parts of the body (including the myocardium). Thus if not attended to, beyond a point, these changes turn into a vicious cycle. A point is reached where the amount and pressure of the blood pumped by heart prove insufficient for day-to-day activities ('heart failure').
Chronic stable angina
In the early stages of CAD, patient might not experience any symptoms at all. CAD and/or IHD at this stage can be diagnosed with only specific tests (discussed later). As the the blood supply through epicardial arteries reduces with disease progression, patient starts experiencing chest discomfort whenever the myocardial oxygen demand starts exceeding the supply limited by restricted blood flow, i.e., during unusual physical exertion (e.g., running or climbing stairs). At this stage, the chest discomfort is reproducible, and the patient can predict its onset based on perceived physical exertion. This condition is known as (chronic) 'stable angina'. It gets relieved upon stopping the physically challenging activity and/or taking drugs that dilate the coronary arteries. The qualifier 'stable' implies that the condition severity of their symptoms and threshold of triggers (physical exertion) remain stable over long durations (say, several months). If unchecked, stable angina eventually progresses to heart failure.
Rapid-onset harms of CAD
The single layer of cells directly coming in contact with blood flowing through an artery are called 'endothelial cells'. They prevent the flowing blood from clotting. While atheromatous plaques tend to be restricted to the artery's wall in earlier stages, in later stages they can rupture through endothelial cells. When this happens, endothelial function is disrupted, and the blood at the site can clot in a process called 'thrombosis'. The resultant clot is called a 'thrombus'. This causes sudden reduction in blood flow and oxygen supply to the portion of the myocardium the artery serves. If blood flow is not restored immediately (typically within 20 minutes), the corresponding cardiomyocytes start dying, and the resulting dead tissue is called an 'infarct'. This event is called a 'myocardial infarction' (commonly known as 'heart attack').
Sometimes a thrombus could be small, and in itself does not cause severe ischaemia, but it could break into smaller pieces called 'emboli' (singular: embolus), which could travel ahead to completely block one of the narrower branches.
Atheromas also produce substances that cause narrowing of arteries, but as long as they remain covered by endothelial cells, these substances are ineffective. However, if the overlying endothelial layer is ruptured, such can enter the bloodstream to cause contraction of smaller blood vessels downstream ('vasospasm'). The resulting symptoms are called 'unstable angina'.
In summary, an atheromatous plaque not only causes largely predictable and gradual damage to the served myocardium by narrowing the artery at its location, but it can also cause somewhat unpredictable, sudden and potentially catastrophic narrowing at the same site (by thrombosis) as well as further downstream (by embolization and causing vasospasm).
There is another type of ischaemia that is not caused by atherosclerosis, i.e., 'variant angina'. Possible cause could include presence of substances causing constriction of coronary arteries that are not produced by atherosclerosis and/or inability of endothelial cells to produce nitric oxide (a substance causing blood vessels to dilate).
Although episodes of unstable angina and variant angina do not individually cause death of cardiomyocytes, if these episodes are too frequent, they could cause remodelling and alter the function of junctional tissue, which in turn could cause (sometimes potentially fatal) arrhythmias.
Any instance of (severe) symptoms (most commonly chest pain / discomfort) related to CAD is known as 'acute coronary syndrome' (ACS). The underlying cause could be an infarct (irreversible) or unstable angina (largely reversible).
Diagnosis of CAD and IHD
Diagnosis of CAD and IHD happens in broadly 2 settings: (a) When we are actively suspecting CAD or (b) incidental diagnosis (without specific reason to suspect its presence).
In vast majority of patients CAD is diagnosed for the first time following first episode of ACS. In these instances fluoroscopy (commonly known as 'coronary angiography', discussed below) is the investigation of choice, which also allows for relieving the occlusion of coronary arteries (e.g., dilatation of the affected segments of coronary arteries and stent placement called 'angioplasty') in the same session.
There are some instances in which CAD is discovered even in asymptomatic patients. These could be when the patient or their doctor are proactive in preventive aspects of health or following pre-anaesthetic work up for major surgery unrelated to the heart.
Investigative tools used in diagnosis of CAD and IHD can be roughly categorized into those that actually show the epicardial coronary arteries and those that do not.
First category of tools that can show epicardial arteries include the following. Typically these techniques can give an estimate of diameter of the arteries' lumen, but they are not good with telling us whether blood actually reaches the myocardium or not.
- Conventional coronary angiography (CAG)
- Coronary computed tomography angiography (CCTA)
- Magnetic resonance imaging (MRI) - only in specific settings
Second category of tools that cannot show epicardial arteries include the following. These techniques directly or indirectly tell us about the health of the myocardium.
- Electrocardiography (ECG)
- Echocardiography
- Myocardial perfusion imaging (MPI)
- Positron emission tomography (PET) with fluorodeoxyglucose (FDG)
- MRI
- Cardiac enzymes
These investigations do not compete with each other, but are rather complementary, i.e., one or more of them might have to be performed to get the most complete possible picture of patient's disease in order to estimate its course as well as determine the best treatment approach.
Following paragraphs will carry a very brief outline of principles, advantages and limitations of all the above investigations. [The order of description roughly corresponds to accessibility and frequency with which these tests are performed]
ECG
Normally the heart muscles in the different parts of 4 chambers contract in a very specific sequence and with specific time gaps. This ensures most efficient pumping action. This synchronized contraction is ensured by junctional tissue. Junctional tissue consists of long thread-like structures travelling within the walls between heart chambers that in turn branch to reach virtually all parts of the myocardium. The junctional tissue transmits very low-voltage electrical signals, in response to which the heart muscles contract. In turn while contracting, the heart muscles also produce their own electrical signals.
ECG measures the electrical signals produced by the junctional tissue as well as the myocardium. These signals are represented as repetitive patterns of 'waves'. Height or depth (amplitude) of the waves corresponds to the strength of electrical signals detected, and the intervening distance between them conveys duration. Any alteration in amplitude of waves suggests reduced functioning of myocardium or altered direction of flow of current.
ECG can also be combined with measurable physical exercise (e.g., treadmill-based 'stress' test) in the hope that it would unmask underlying myocardial ischaemia which would typically be asymptomatic at rest. Also, ability to endure high level of physical stress ('exercise tolerance') is an independent indicator of good cardiac health.
ECG is inexpensive, easy to setup and non-invasive, which are its advantages.
ECG (especially resting ECG) can be poor at predicting underlying ischaemia.
Echocardiography
Echocardiography (often shortened to '2D-Echo') is a kind of ultrasonography that involves sending ultrasonic sound waves towards heart and sensing their reflections. Ultrasound waves are very high-frequency sound waves that cannot be heard by the human ear.
Through echocardiography, we can see size, shape and motion of chambers of heart and their thickening. And from that we can calculate LV 'ejection fraction' (LVEF). LVEF is one of the indicators of forcefulness of contraction of LV. Using 'Doppler' principles, velocity of blood flow and its turbulence can be gauged. Echocardiography can also be combined with exercise or pharmacological (drug-based) stress testing.
Echocardiography is also relatively inexpensive, non-invasive, and does not require much preparation.
Echocardiography is highly operator-dependent, meaning acquisition as well as interpretation of images is dependent on the doctor performing it. Presence of intervening structures like ribs between the probe and the heart can make interpretation difficult. The posterior parts of heart are not seen well through (the commonly performed) transthoracic echocardiography.
Cardiac enzymes
Damage to the individual cardiomyocytes happens during severe ischaemia or when they die because of it. Whenever this happens, proteins that are contained almost exclusively in the cardiomyocytes (and not in other cells of the body) leak into the blood. These proteins are called 'cardiac enzymes'. So, elevated blood levels of cardiac enzymes act as confirmatory for severe and/or irreversible damage to the myocardium.
CAG
CAG involves advancing a metallic guidewire with J-shaped tip up to the root of the aorta, and over which a polymer ('plastic') based long tube called 'catheter' is slid. This catheter is introduced into the holes (ostia) of the initial segments of the major coronary arteries in a process called 'cannulation'. Following this, a radiopaque dye is injected, which delineates the outlines of lumens of the cannulated artery and its branches. Segments of these arteries that appear to be starkly narrower are considered to be having atherosclerotic plaques. Ratio of the shortest diameter to that of the widest diameter is expressed as 'percent of occlusion'.
Catheter access to the large epicardial arteries enables few additional medical applications.
- The catheter tips allow for measurement of pressure, which in turn allows for quantification of pressure drop across a stenosis.
- Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) probes attached at the leading end of catheter can give some idea of the arterial wall (as against just the outline of the lumen).
- Most important benefit of catheterization as part of CAG is that in the same session, apparent stenosed segments of arteries can be dilated and fit with metallic stents (that prevent future narrowing of the dilated segments). These and related procedures aimed at improving the width of coronary arterial lumen are known as 'coronary angioplasty' or 'percutaneous coronary intervention' (PCI).
Most of the limitations of CAG arise from the fact that it only shows outline of the arterial lumen, and not the wall itself, and are discussed below.
- Non-visualization of collateral blood vessels. Even normal hearts have narrow arteries that act as connections between parallelly running arteries. These are known as 'collateral' arteries. When the proximal (initial) part of an artery becomes gradually narrow, collateral blood vessels connected to its distal (later) part become wider to reduce / nullify the effect of proximal narrowing. While CAG can show larger collateral arteries, it cannot show smaller ones thus making us overestimate the significance of CAD.
- Underestimation of narrowing. Atherosclerosis is a diffuse process, meaning, many times it leads to uniform narrowing of a fairly long segment of an artery. And as the vessel wall cannot be seen as part of CAG, such a diffuse narrowing can be misconstrued to be 'normal'.
- Overestimation of narrowing. This can happen in 2 different instances.
- Missing the wider dimension of lumen. CAG is essentially a 2-dimensional imaging modality, and arteries are seen from a side profile. So, if a plaque is mainly along the upper and lower walls, and not along the sides, the effective diameter of the lumen could be greatly underestimated.
- Not accounting for 'positive remodelling'. An atherosclerotic plaque in the initial stages weakens the arterial wall, and the involved arterial segment becomes wider because of the high pressure of blood flowing through it. When this happens, a normal or minimally narrowed segment of artery can appear paradoxically narrower than it actually is. This results in overestimation of severity of its occlusion.
- Variation of artery lumen by other factors. Nitroglycerin is used as part of CAG that widens the (smaller) coronary arteries, so the diameter of blood vessels measured under its influence could be different from that in the natural state. Width of coronary arteries varies with the rate of blood flow through them, and it could be significantly different from 'normal' times during the CAG procedure. The iodine-based dye used for CAG can cause reflex constriction of some of the coronary artery segments. The catheter material is recognized as foreign body by the endothelium, and contact with it can alter diameter of the receiving artery.
- Wrong estimation of length of stenosis is also possible because of aforementioned reasons.
- Inability to visualize intramural arteries. The intramural arteries that are much closer to the cardiomyocytes cannot be visualized by CAG. It is possible for these blood vessels to also have atherosclerotic narrowing. So, presence of IHD in apparent absence of CAD (as adjudged by CAG) is known as 'coronary syndrome X' or 'microvascular disease'.
- The greatest limitation of CAG is that it gives very little to no information about the adequacy or inadequacy of blood supply to the myocardium served by the apparently stenosed coronary arterial segments. Hence, it is possible that the corresponding myocardium would be well perfused and normally functioning eliminating the need for angioplasty. Conversely, the cardiomyocytes served by a stenosed epicardium could be irreversibly dead (non-viable) making angioplasty (and bypass surgery) useless.
CAG is a relatively safe procedure, and chances of complications increase with severity of underlying CAD and IHD. Frequency of complications is less than 2%. Frequency of serious complications like stroke or heart attack is less than 1%. Frequency of death is ~0.1%. In general, complications are more likely and more severe in those with already established CAD, IHD and associated disorders like uncontrolled and long-standing hypertension and diabetes. Salient complications of CAG are as follows.
- Allergic reaction to the dye.
- Local complications in and around the artery chosen for inserting the guide wire (e.g., in the groin or wrist regions).
- (Usually transient) worsening of the kidney function. Risk is greater in those with already impaired kidney function (and can range from 12 to 50%).
- Arrhythmias.
- Injury to the coronary arteries. Its frequency is low, but is a serious complication that could require emergency cardiac surgery.
- Myocardial infarction (also rare).
CCTA
CCTA involves acquiring a CT scan of the heart region 10 to 15 seconds after injection of iodinated dye into one of the limb veins. This dye is similar to that used in CAG in that it is radiopaque and blocks X-rays. Compared to CAG, it gives additional information about length of plaques and their composition.
A similar scan called 'coronary artery calcium' (CAC) testing is used to detect and estimate amount of calcium in atheromatous plaques. Presence and severity of calcium in plaques predicts worse outcomes.
Combined findings of CCTA and CAC help in determining need for urgent attention. They also help in determining long term treatment strategy.
Limitations of CCTA
- The iodinated dye used for the study carries the same risks as mentioned in the section on CAG.
- Calcification within atherosclerotic plaques is irreversible, so the CAC score does not improve even after improvements in plaque stability and myocardial perfusion.
- CCTA tends to overestimate the likelihood of obstructive CAD.
- Extensive calcification tends to produce blooming artifacts, which interfere with the ability to visualize the lumen and estimate the severity of stenosis.
- It cannot detect stenosis of intramural arteries (coronary syndrome X or microvascular disease).
Myocardial perfusion imaging (MPI)
MPI can be performed using single-photon emission computed tomography (SPECT) or positron emission tomography (PET). Tracers used for MPI dissolve uniformly throughout the blood. Their uptake by the cardiomyocytes depends on 2 factors: (a) rate of blood flow / perfusion, (b) and avidity of uptake by cardiomyocytes. While PET provides better image quality, tracers used for PET imaging have very short half-lives, and hence, are not available easily. Hence, present discussion is restricted to SPECT tracers.
'Sestamibi' (or MIBI) and 'tetrofosmin' are the 2 major SPECT tracers used for MPI. They are both positively charged (cationic). When cardiomyocytes use fatty acids and glucose under sufficient oxygen availability, the inner part of organelle called 'mitochondrion' develops a relative strong negative electrical potential, because of which these cationic tracers get trapped within the mitochondria. Portions of myocardium experiencing ischaemia (and hence, hypoxia) take up less of the tracer as they would have switched to anaerobic glycolysis (that does not develop negative electrical potential within the mitochondrion). Portions of myocardium consisting of dead cells or scar tissue do not take up any tracer. These tracers are tagged with radioactive technetium-99m, and hence can be detected on a gamma camera (SPECT scanner). Thus dead or ischaemic myocardial tissues appear as 'cold spots' compared to the better perfused parts.
Coronary blood flow at rest is 0.7 to 1.0 mL/min per gram of myocardial tissue, which can increase 3 to 5 times during maximum stress because of dilatation of intramural arteries. However, the stenosed coronary arteries show no or negligible increase in blood flow in response to stress as the corresponding intramural arteries would be already maximally dilated at rest. Thus, if the tracers are injected to a patient with CAD at maximum stress, the disparity in tracer uptake between the better perfused and ischaemic parts of the myocardium would be further stark than at rest alone (see figure below).
| Normal | Stenosis | |
|---|---|---|
| Rest |

|
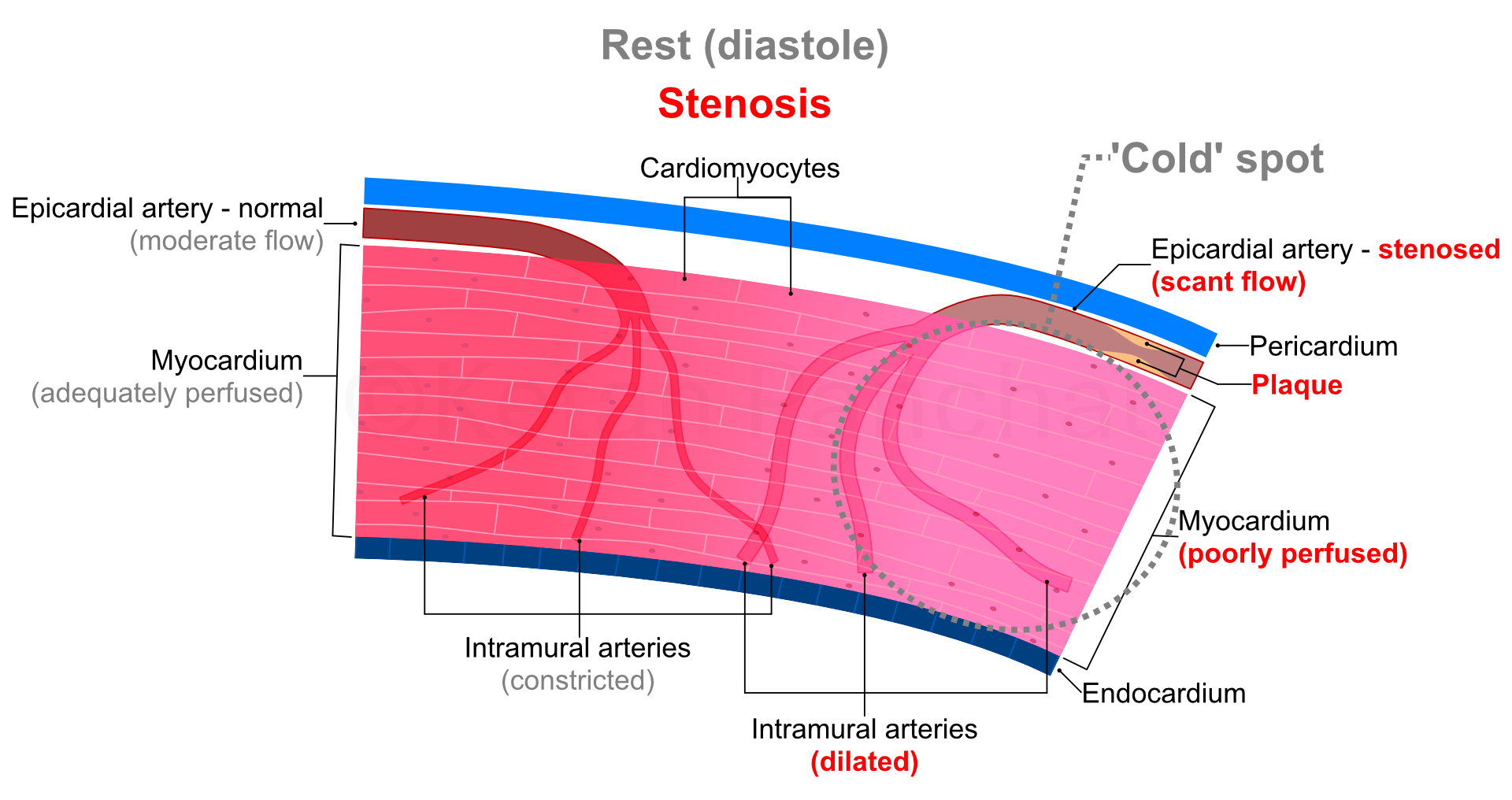
|
| Stress |

|
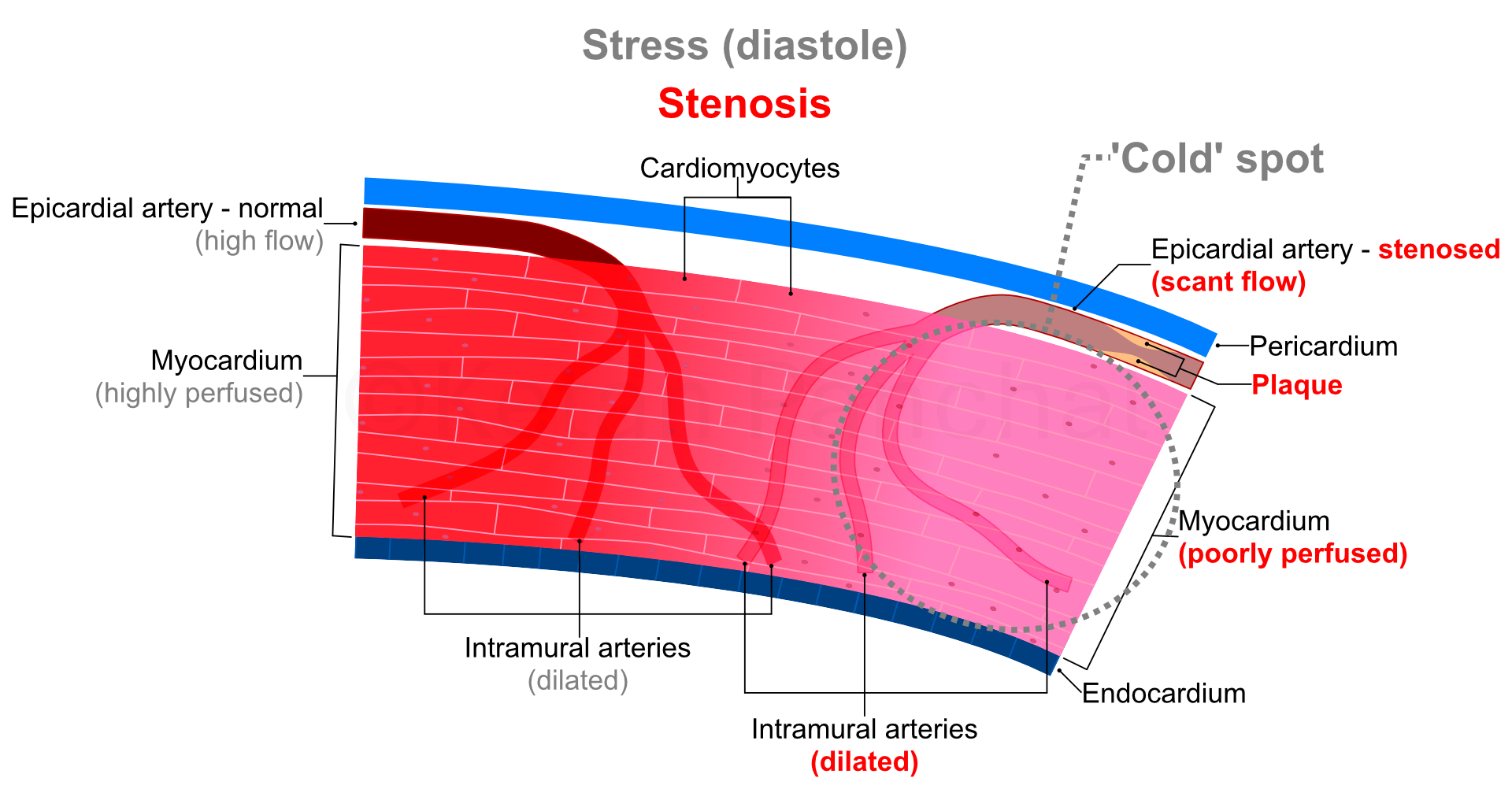
|
The ischaemic tissue emits no or fewer photons (i.e., is 'photopenic' or 'cold') only during stress, but emits normal or near-normal amount of radiation during rest image. This is known as 'reversible' or 'inducible' perfusion defect. On the other hand, dead or unviable or scarred myocardium does not emit photons even in rest image, and thus is recognized as a 'fixed defect'.
In most instances, apart from looking at the intensity of tracer uptake, 'ECG-gated' SPECT acquisition allows for assessing heart wall motion and thickening, which in turn allow for calculation of LVEF. These data points help form a more comprehensive impression of multi-vessel CAD, wherein otherwise MPI uncovers inducible ischaemia only in the most severely affected myocardial territories.
Limitations of MPI SPECT
- MPI SPECT delineates relative ischaemia rather than absolute, i.e., myocardium that is ischaemic but less so compared to the rest of the myocardium would show up as 'normal'.
- There can be attenuation of the inferior wall of LV in obese patients.
- The scanning procedure and waiting duration are both much longer than in other imaging studies. Hence, this procedure is typically not performed in emergency setting.
- Availability is more limited compared to other imaging studies.
Cardiac FDG PET
Most of the aforementioned investigations only provide indirect evidence of 'alive' status of cardiomyocytes. If a myocardial territory experiences chronic ischaemia, the corresponding cardiomyocytes become less active with accompanying reduction in blood flow even in absence of obstruction. This state is known as 'chronic hibernation'. Hibernating myocardium can be mistaken for dead tissue on other procedures like echocardiography and MPI. However, if blood supply to such tissue is increased it can regain function and thus reduce mechanical workload of rest of the better performing myocardium.
Fluorodeoxyglucose (FDG) is a glucose analogue that is taken up by cardiomyocytes. Normally functioning as well as hibernating myocardium takes it up, whereas scarred or dead myocardial tissue does not take it up. FDG uptake can be assessed using cardiac FDG PET, which is discussed in much details elsewhere. However, FDG cardiac PET cannot cannot reveal inducible ischaemia. Hence, cardiac FDG PET is used for 'viability assessment'.
Cardiac MRI
Cardiac MRI is performed in 2 major settings. Conventionally, cardiac MRI can be used to delineate scarred tissue from viable tissue.
A type of MR angiography helps look at blood supply, but it is available at only very specialized facilities.
Treatment of CAD / IHD
Treatment of CAD and IHD depends on multiple factors. Low- and intermediate-risk patients could be treated with only medical therapy (lifestyle changes and drugs), wherein modifiable risk factors like smoking, obesity, diabetes, hypertension, blood lipid levels, etc. are attended to. Higher risk patients and/or those presenting as emergency cases are managed by a revascularization procedure (in addition to medical therapy). 'Revascularization' refers to attempt to physically restore normal blood flow across stenosed segments of coronary arteries, and is done through PCI or coronary artery bypass graft (CABG) surgery.
PCI involves dilatation of stenosed blood vessel with a balloon attached to a catheter, which is usually followed by placing a metallic stent that prevents the blood vessel walls from collapsing.
CABG is a major surgery, and involves connecting one of the larger non-coronary arteries to the LAD with a segment of artery or vein harvested from some other part of the patient's body.